Platform optimization for efficient AAV purification: insights from a CDMO
Vincent Ravault (Yposkesi) & Nicolas Laroudie (Thermo Fisher Scientific).
Cell & Gene Therapy Insights 2022; 8(1), 1–14
DOI: 10.18609/cgti.2022.001
Over the last decade, the number of clinical trials involving recombinant adeno-associated viral (AAV) vectors has dramatically increased, the diversity of serotypes has expanded, and the demand for larger quantities of highly purified material manufactured to cGMP standards has rocketed. For contract development and manufacturing organizations (CDMOs) like Yposkesi, the manufacturing challenges are centered around flexibility, robustness, and productivity, especially with regards to purification. Universal tools able to address any serotype with minimal process adjustments are critical. In this article, we describe how POROS™ CaptureSelect™ AAVX resin can be used as a pan-affinity tool for the universal capture of AAV vectors, and how Yposkesi optimized the operational parameters to make the resin an efficient, robust, and productive purification platform that fits within the constraints faced by CDMOs.
As a full-service CDMO for innovative gene therapy products, Yposkesi supports customers from early-stage development, including process and analytical development, through to large-scale production and commercial supply of gene therapy products. Yposkesi produces recombinant adeno-associated virus (rAAV) and recombinant lentivirus (rLV) vectors using adherent- and suspension- adapted cell platforms. The manufacturing platform at Yposkesi currently includes four independent production suites equipped with 200 L single-use bioreactors, which will evolve to include a 1000 L single-use bioreactor from 2023. Yposkesi is currently building an additional 5,000 m2 clinical/commercial manufacturing plant to support the growing demand for viral vector supply. This article describes how Yposkesi developed an AAV purification platform for a range of serotypes based on Thermo Fisher Scientific’s POROS CaptureSelect AAVX Affinity Resin.
Yposkesi’s AAV manufacturing process
The established AAV manufacturing process at Yposkesi is shown in Figure 1.
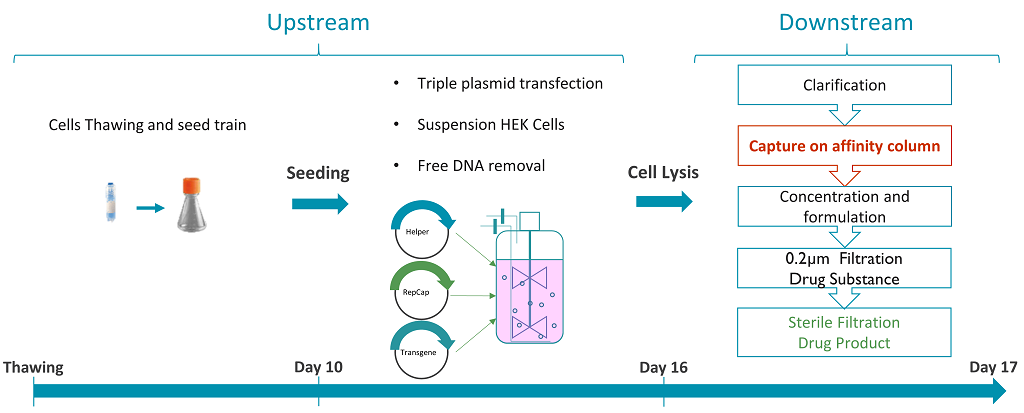
Figure 1: AAV manufacturing process at Yposkesi.
AAV vectors are produced by triple plasmid transfection in human embryonic kidney (HEK) cells. The lysate is clarified and then directly loaded onto an affinity column. The eluted vectors are concentrated and formulated, before being sterile filtered. The full process lasts 17 days, from cell thawing to drug product filling. The current AAV purification process involves the use of different affinity sorbents according to the AAV serotype to be produced. The POROS CaptureSelect AAVX Affinity Resin leans on the use of a ligand derived from a heavy-chain antibody that can bind AAV serotypes 1–9 and synthetic or recombinant AAV vectors, offering a great opportunity to develop the next AAV purification platform at Yposkesi.
Evaluation of dynamic binding capacity
As a first step to evaluate the AAVX resin as a platform purification solution, the dynamic binding capacity was evaluated using an AAV8 serotype. The binding capacity was assessed using 1 mL-prepacked columns, packed with either POROS CaptureSelect AAV8 or POROS CaptureSelect AAVX. The binding capacity was assessed at 1 and 3 mins residence time on two different feedstocks, each with different initial virus titers. Clarified supernatant containing AAV8 vectors was directly loaded on the affinity columns until a 10% breakthrough in AAV8 was observed in the flowthrough. Multiple fractions (column volumes [CV]) were collected at the outlet of the column during the loading phase, and the quantity of capsids was determined by ELISA assay in each collected fraction. The results for the 3 mins residence time are presented in Figure 2.
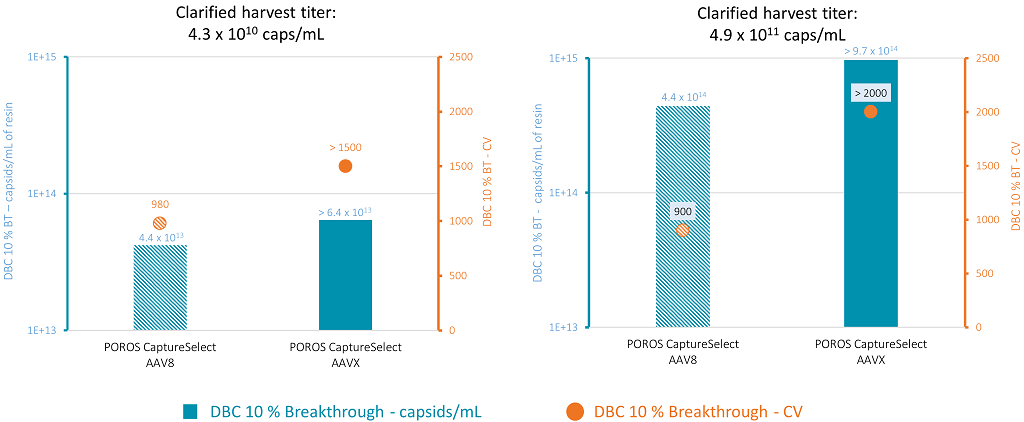
Figure 2: Binding capacity at 3 mins residence time and 10% breakthrough for POROS CaptureSelect AAV8 and POROS CaptureSelect AAVX at AAV titers of 4.3×1010 caps/mL (left) and 4.9×1011 caps/mL (right). The blue bars represent the binding capacity in terms of capsids per mL of resin at 10% breakthrough. The orange dots represent the column volumes that lead to 10% breakthrough.
No breakthrough was observed on the AAVX resin at loading volume of up to 1,500 or 2,000 column volumes for the low viral titer and higher titer feedstock, respectively. Both resins showed higher binding capacity when feeds contained a higher vector titer, but overall, the AAVX resin showed a higher binding capacity for AAV8 than the Poros CaptureSelect AAV8 resin.
Figure 3 shows the binding capacity of AAV8 vectors measured at 1 min residence time on both resins, showing similar binding capacities compared with the 3 mins residence time.
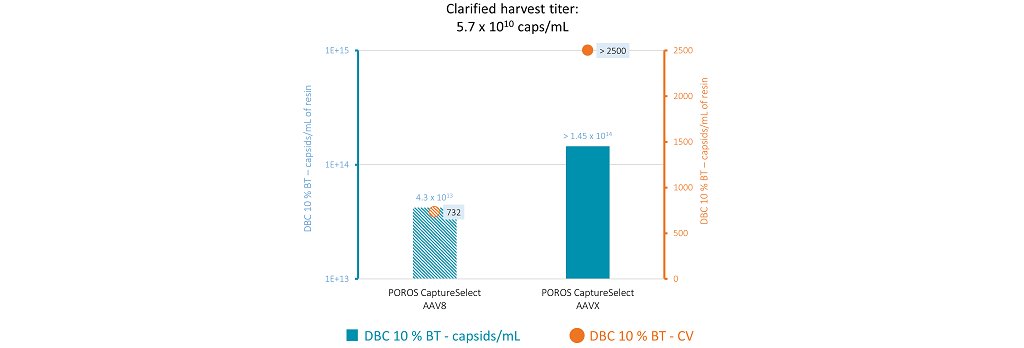
Figure 3: Binding capacity at 1 min residence time and 10% breakthrough for POROS CaptureSelect AAV8 and POROS CaptureSelect AAVX at an AAV titer of 5.7×1010 caps/mL. The blue bars represent the binding capacity in terms of capsids per mL of resin at 10% breakthrough. The orange dots represent the column volumes that lead to 10% breakthrough.
At both residence times, there was no breakthrough on AAVX, with loading volumes up to 2,500 column volumes (CV). The results from these binding capacity studies led to three main conclusions:
1. The AAVX resin has a better AAV8 binding capacity than the AAV8 resin
2. Binding capacity increases with harvest titer
3. Residence time has no significant effect on the binding capacity
Defining operating conditions for purification of AAV8 & AAV2
The operating conditions for the capture of the AAV8 serotype were defined according to the DBC data obtained previously. Screening of capture conditions was performed on 1 mL-pre-packed columns with AAV8 or AAVX resin (Figure 4). The material loaded onto the columns was a clarified supernatant containing AAV8 vectors.
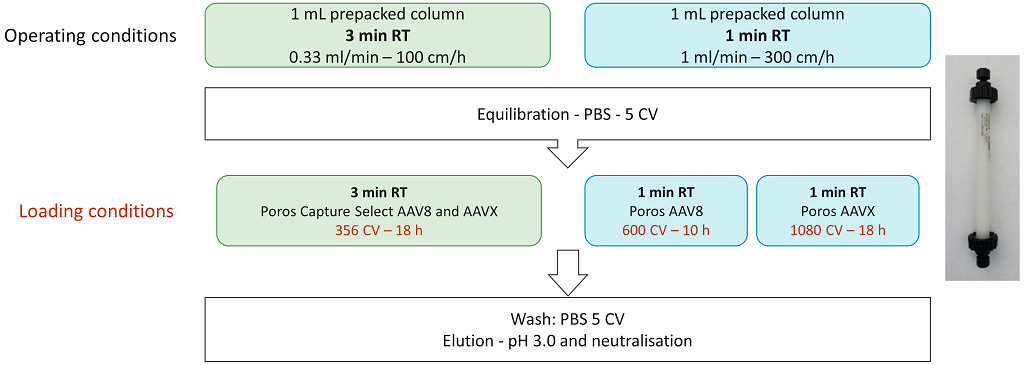
Figure 4: Experimental plan for the definition of operating conditions for purification of AAV8.
To align with our AAV manufacturing operating conditions, the maximum loading time selected was 18 hours – (overnight loading). Two residence times were evaluated: 3 mins and 1 min. The loading volumes selected were 356 CV (for AAV8 and AAVX) with a 3 mins residence time, and 600 CV (AAV8) and 1080 CV (AAVX) with a 1 min residence time. These CVs are all below the resin binding capacities at 10% breakthrough defined earlier (Figures 2 & 3). After loading and washing, purified product was recovered during the elution step at low pH and was immediately neutralized.
The clarified harvest and eluent were tested for viral genome (VG) titer. Similar quantities of AAV vectors were loaded on the AAV8 and AAVX resins at 3 mins residence time. As shown in Figure 5, the quantity of AAV8 vector recovered after elution and the AAV8 yield was very similar for both resins. The resins showed no significant difference in performance when loading at 3 mins residence time or at 18 hours loading time.

Figure 5: AAV8 capture conditions – results for 3 mins residence time. The blue bars represent the product quantity loaded on each column. The orange bars represent the quantity of purified product recovered during the elution. The red dots represent yield.
Results at 1 min residence time are shown in Figure 6.
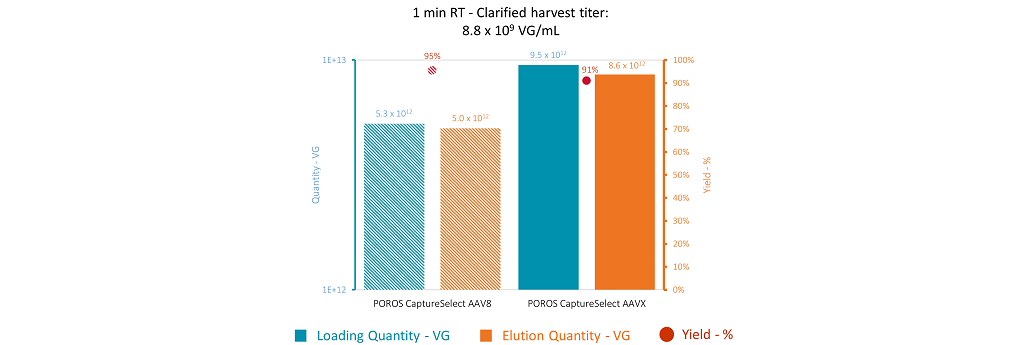
Figure 6: AAV8 capture conditions – results for 1 min residence time. The blue bars represent the product quantity loaded on each column. The orange bars represent the quantity of purified product recovered during the elution. The red dots represent yield.
As a consequence of the different binding capacities at 1 min residence time, the loading times were different for the two sorbents – 10 hours for the AAV8 resin and 18 hours for the AAVX resin. Thus, the total quantity of AAV8 capsid loaded on the resins was around 1.8 times higher for the AAVX resin compared with the AAV8 resin. As a result, the quantity of purified recovered product for AAVX was approximately 1.7 times higher. The step yields for both resins were also very similar and close to 90% which is higher than the yield of around 70% obtained with a residence time of 3 mins. These results indicate that it will be possible to switch from POROS CaptureSelect AAV8 to AAVX for the purification of AAV8 serotype. Based on these results with POROS CaptureSelect AAV8, the AAVX resin was also evaluated for the capture of another serotype of AAV: AAV2 (Figure 7).
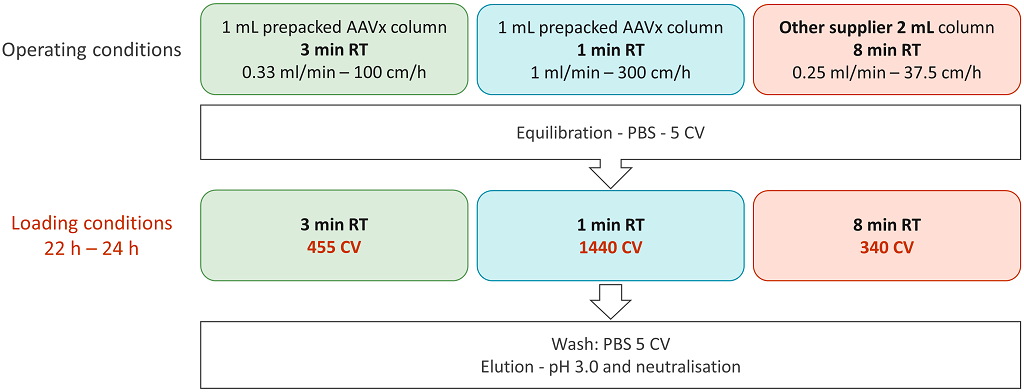
Figure 7: Experimental plan for the definition of operating conditions for purification of AAV2.
The aim was to compare POROS CaptureSelect AAVX with an affinity resin from another supplier, which is currently used at Yposkesi for AAV2 processes. The screening of the capture conditions was performed on 1 mL prepacked columns. Two residence times were applied for the AAVX resin: 3 mins and 1 min. The residence time applied to the other affinity resin was 8 mins, according to supplier’s recommendation. Three purification conditions were screened for the capture of the AAV2 vector. At 3 mins residence time, the volume of clarified harvest loaded on the column was 455 CV, whereas at 1 min residence time the volume loaded on the column was 1,440 CV. The same starting material was used for all trials. For the other resin, only 340 CV were loaded since the residence time applied was higher. After column washing, the product was eluted at low pH, and the loading and elution fractions were tested for VG titer. Using the AAVX resin and decreasing the residence time from 3 min to 1 min resulted in an increase in VG yield from 57% to 89% (Figure 8).
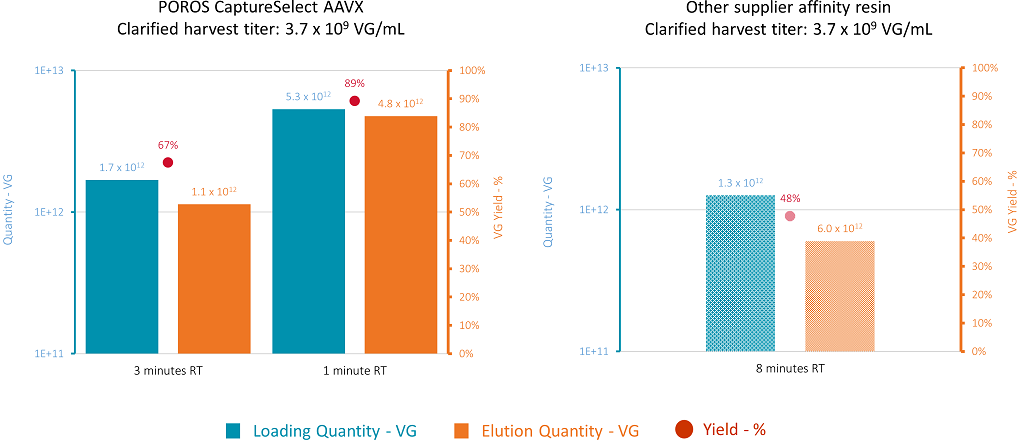
Figure 8: Definition of the operating conditions for the purification of AAV2 using POROS CaptureSelect AAVX (left) or alternative supplier’s affinity resin (right). The blue bars represent the product quantity loaded on each column. The orange bars represent the quantity of purified product recovered during the elution. The red dots represent yield.
Using the affinity resin from another supplier with higher residence time (8 mins, imposed because of the compressibility of the media, and as recommended by the supplier), resulted in a low volume loaded on this column. The AAV2 yield is significantly lower than the yield obtained with AAVX: 48% yield, versus 70–90% yield obtained with AAVX. This part of the study demonstrated that using a lower residence time results in higher AAV binding capacities for both Thermo Fisher Scientific resins, and that the AAVX resin shows better results for the capture of AAV8 and AAV2 vectors. The volumes of clarified harvest that can be loaded on AAVX without any AAV breakthrough in the flowthrough are 1080 CV for AAV8, and 1440 CV for AAV2. The promising results obtained with POROS CaptureSelect AAVX led us to select this resin for the next part of the study and to work with a residence time as close as possible to 1 min.
Scale-up of the chromatography step
The experimental conditions determined using AAVX for the capture of AAV2 and AAV8 vectors were adapted for the purification of clarified harvest from a 10-liter bioreactor (Figure 9).
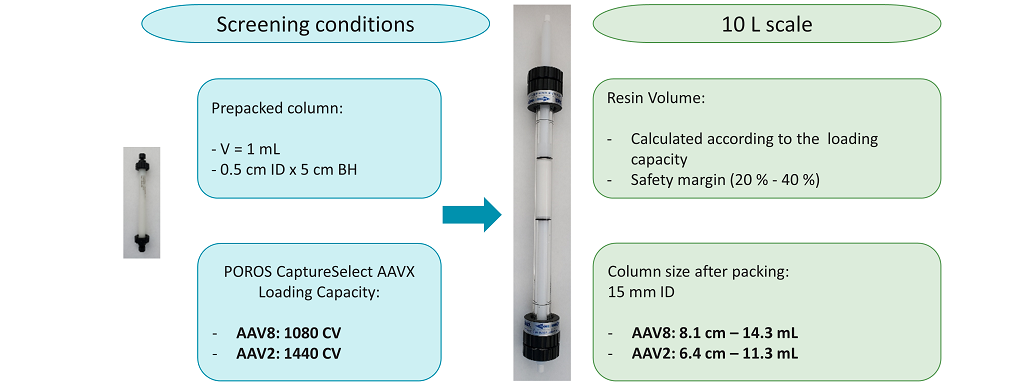
Figure 9: POROS CaptureSelect AAVX chromatography scale up. Conditions to be tested are listed for small-scale (left) and 10-liter scale (right).
The volume of resin necessary to purify a 10 L clarified harvest was calculated by applying the column loading capacity in terms of CV determined previously during the screening for AAV8 and AAV2 processes. This AAVX resin volume was found to be 14.3 mL for AAV8 capture and 11.3 mL for AAV2 purification process. AAVX resin was packed in a 15 mm internal diameter glass column, which allowed for a resin bed height that would be easily transferrable to GMP scale. The column bed height was 8.1 cm for AAV8 purification and 6.4 cm for AAV2 purification. In order to obtain the starting material for resin evaluation, two 10 L bioreactors were used to produce AAV2 and AAV8 vectors from HEK cells. After AAV production, cells were lysed, and the lysate was clarified and filtered using a 0.22 μm filter. After lysate filtration, the pool titer was 1.10 x 1011 VG/mL for AAV8 vectors and 3.70 x 109 VG/mL for AAV2 vectors. The selected operating conditions for the AAVX resin to purify AAV8 and AAV2 from a 10 L clarified harvest are shown in Figure 10.
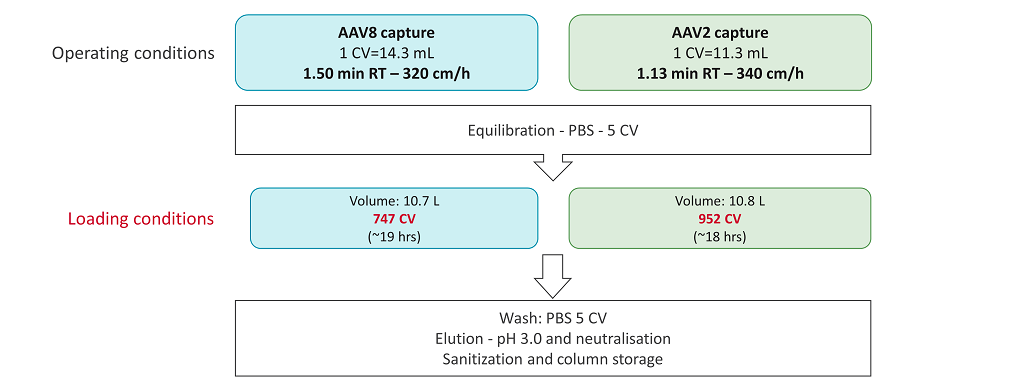
Figure 10: Selected chromatography operating conditions at larger scale.
The residence time for both AAV processes was close to 1 min. 747 CV of clarified harvest were loaded on to the AAVX resin for AAV8, and 952 CV for AAV2, while the loading times were in the same range. The purified products were recovered during elution at low pH and then neutralized. VG titers, total protein content, and residual DNA levels were assayed in the clarified harvests (starting materials) and in the elution fractions. The pressure was monitored at the inlet of the column during the loading step for the AAV8 and AAV2 capture process. The pressure slightly increased during the loading stage but stayed within an acceptable range. The pressure was around 1.5 bars at the end of the loading step, which helps to provide good conditions for a transfer to GMP scale. Even though VG titers in the starting material were very different for the AAV2 and AAV8 serotypes, the final yields of the capture step are close to 100% for both serotypes and there was good scalability from lab scale development to the 10 L scale (Figure 11).
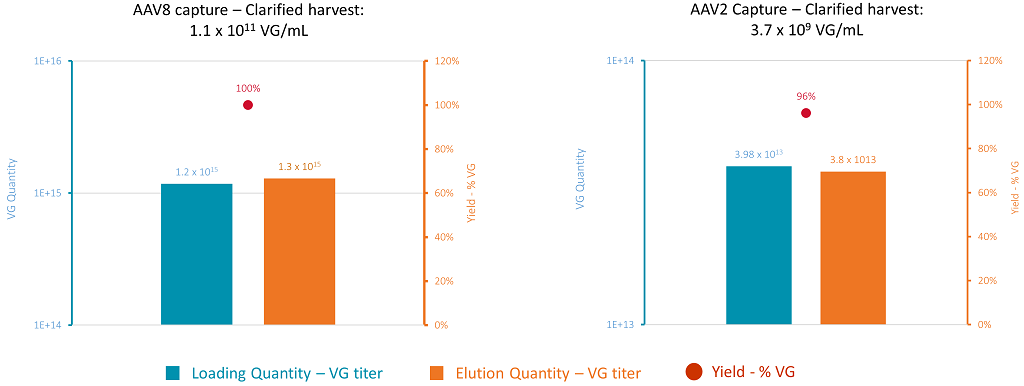
Figure 11: AAV capture on POROS CaptureSelect AAVX – yields for AAV8 (left) and AAV2 (right). The blue bars represent the product quantity loaded on each column. The orange bars represent the quantity of purified product recovered during the elution. The red dots represent yield.
Additional experiments revealed that the purity of AAV vectors captured with AAVX resins appears to be very high. There was an impurity reduction of over 99% in the purified product after capture on AAVX for each serotype. This clearance rate could be even further optimized by adding an intermediate washing step or implementing a polishing column after the AAV capture step.
Conclusions
This long-term study with POROS Capture- Select AAVX resin has highlighted several advantages of AAV capture using this resin compared to other affinity resins commercially available:
– Flexibility in terms of serotypes: capture of AAV1 to AAV9 serotypes and synthetic and recombinant serotypes;
– Possibility to standardize a purification platform for several AAV serotypes with only a few adjustments;
– Cost reduction due to shorter residence times and very high loading volumes;
– Low level of impurities captured on the resin. This could be further optimized for each serotype if needed (wash conditions screening or addition of a polishing step);
– Good scalability of the downstream platform. It is compliant for a large-scale GMP AAV manufacturing process.
Overall, Yposkesi concluded that the POROS CaptureSelect AAVX resin appears to be a great tool to improve purification processes in terms of quality, cost, and standardization. Yposkesi plan to implement this resin for the purification of other AAV serotypes.
Still interested about the topic?
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Thermo Fisher Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: This article is a transcript of a webinar, which can be found here.
Webinar recorded: Oct 26 2021; Revised manuscript received: Jan 4 2021; Publication date: Jan 24 2022.


