Improvement of adeno-associated viral vector Production using “HEK+” cells, deleted of SV40 large T antigen encoding sequences
S. Charrier1, E. Triebe2, J. Cheuzeville2, L. Suarez1, O. Moses1, F. Amor3, N. Avenier1, C. Rousseaux1, M. Amendola3, P. Santambien2, B. Mullan1 and S. Martin2
1- Yposkesi, an SK pharmteco company, 26 rue Henri Auguste-Desbruères, 91100 Corbeil-Essonnes, France; 2- Généthon, 1 bis rue de l’internationale, 91000 Evry, France; 3- Integrare, UMR S951, Généthon, 1 bis rue de l’international, 91000 Evry, France.
The majority of in vivo gene therapy clinical trials require the production of recombinant Adeno-Associated Virus (rAAV) vectors with high purity and potency. We have previously isolated a high-producing clone derived from the human embryonic kidney (HEK) 293T cell line which grows in suspension in serum-free media, but the presence of the SV40 large T antigen may pose safety concerns. We have recently generated a new clonal cell line (called HEK+) derived from the HEK293T clone by removing the SV40 T antigen-encoding sequences via CRISPR-Cas9 genome editing. In this study, we have evaluated the performance of this cell line. Firstly, regardless of AAV serotypes (2, 6, 8, or 9), we have observed a two-fold increase in AAV productivity in comparison to the parental HEK293T clone in Shake Flasks (100mL) and also in 10L bioreactors, with titres higher than 1E11 VG/mL in the crude lysate. Secondly, we have evaluated the stability of this cell line for rAAV vector productivity up to 39 cell passages by measuring E1A and E1B mRNA expression. Finally, we have produced rAAV2/8 vector with a transgene of interest at the 50L bioreactor scale and confirm the ability to obtain high titres of purified rAAV vectors without SV40 T antigen expression.
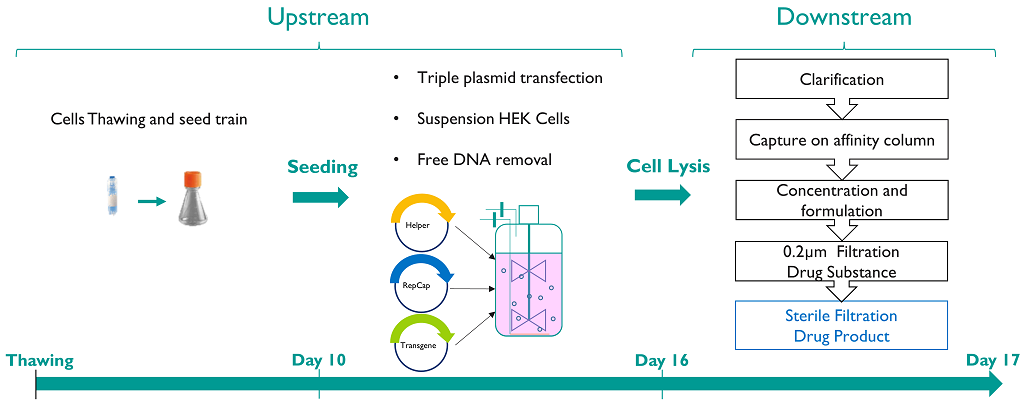
rAAV Process
The assembly of rAAV vectors requires:
- The recombinant vector genome composed of the gene of interest (GOI) and the regulatory elements for the transgene expression in target cells (promoter, poly A, introns…) flanked by inverted terminal repeats (ITRs);
- The AAV rep and cap genes provided in trans;
- The helper functions from Adenovirus for efficient replication and rescue of the recombinant genome.
rAAV vectors are produced by triple plasmid transient transfection in HEK293T cells (parental or HEK+ clone). After benzonase and lysis treatment, the crude lysate is clarified and loaded onto an affinity capture chromatography column. Then the eluted vectors are concentrated and formulated, before being sterile filtered to obtain the drug substance (DS).
Productivity of rAAVs with HEK+ cells
Small scale
For cell expansion, cells were thawed and maintained in a serum-free medium at 37 °C in a humified 5% CO2 atmosphere. The average specific growth rate (µ) was 0.033 h-1 and the cell doubling time (td) was 21.1 ± 1.1 h. Three runs of rAAV were produced in triplicate at 100mL scale in 250 mL shake flasks in order to test several AAV serotypes (2, 6, 8 and 9).
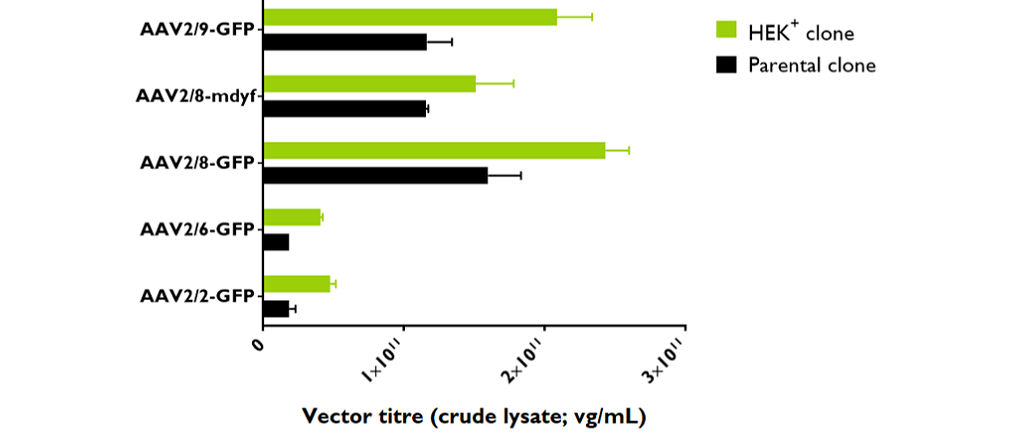
The productivity of the HEK+ cells, based on rAAV qPCR titre, was significantly higher, regardless of AAV serotypes, in comparison to the parental HEK293T clone.
Large scale
rAAV were produced at 10L scale in BioFLO® 320 bioreactor (BR 10L) (Eppendorf) or at 50L scale in STR bioreactor (BR 50L). The bioreactor settings were similar to the parameters used for the parental clone. Cells were transfected at a final cell density ranging between 1.8E6 and 3E6 cells/mL. Vector Titres in crude lysates were determined by qPCR. After clarification, the crude lysate from 50L was purified by AAV8 POROS™ affinity chromatography (Thermo Scientific™). The eluted product was formulated after concentration by flow tangential filtration with hollow fiber.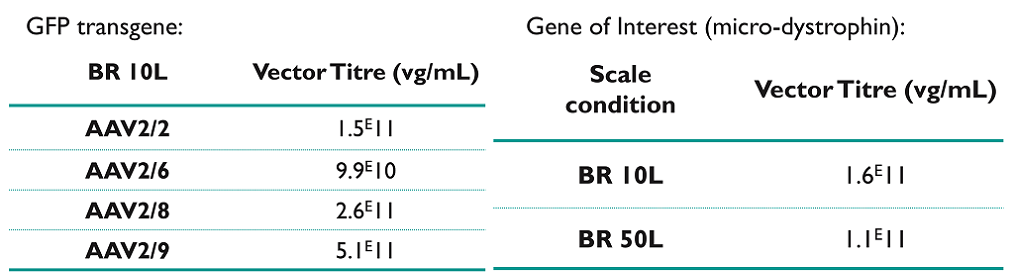
The rAAV productivity from HEK+ cells at large scale was higher than the results obtained in Shake flask, with a titre ≥ 1E11 vg/mL (crude lysate) depending on the serotype.
After rAAV purification and concentration of final DS, the global yield was around 41% and the DS titre was equal to 6.E13 vg/mL for a final DS volume of 29.8 mL. Downstream purification yields were comparable to those previously obtained from the parental cell line.
Stability of HEK+ cells up to passage 39
Absence of large T antigen expression
In HEK293T cells, we have confirmed by Targeted Locus Amplification (TLA) the presence of SV40 T antigen expression cassette located on chr 3 and by qPCR that 2 copies of SV40 T antigen are integrated.
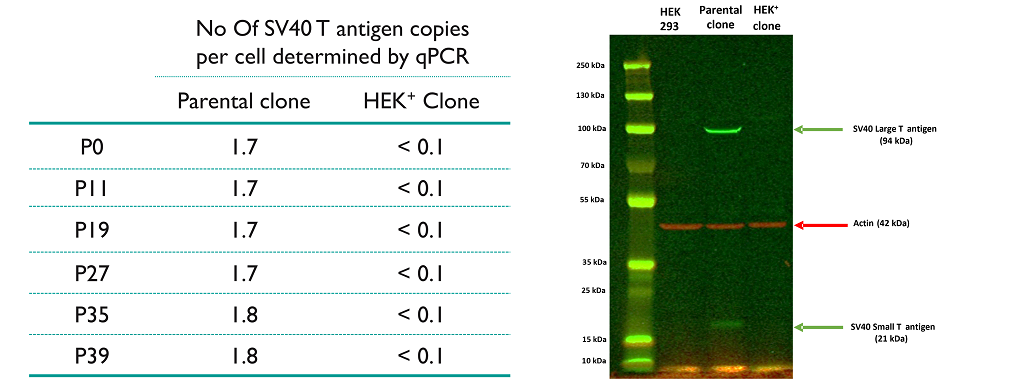
In HEK+ cells, the deletion of genes coding for SV40 small and large T antigen results in a detection limit lower than 0.1 copy per cell by qPCR and an absence of protein expression by Western-blot and ELISA (data not shown). The parental HEK293T clone was evaluated as positive control and HEK293 cells as negative control.
E1A and E1B expression
E1A and E1B expression are essential helper factors for AAV manufacture. HEK293 cell line was immortalized by the integration of a ~4.3 kbp adenoviral 5 (Ad5) genome fragment containing the E1A and E1B genes, located on chr. 19.
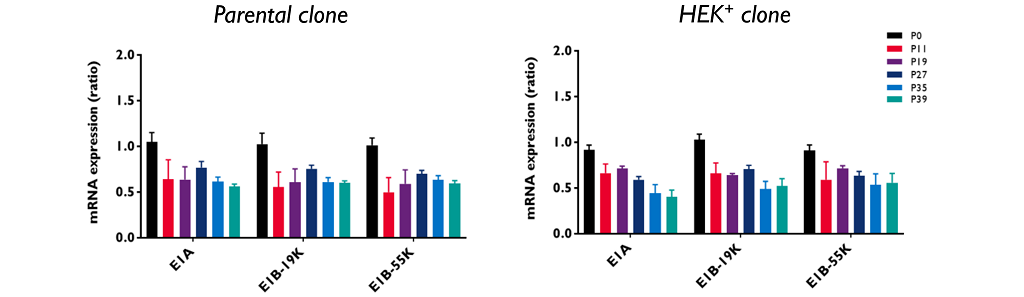
The E1A and E1B mRNAs were evaluated by RT-ddPCR and show a similar expression profile in the parental clone and in HEK+ cells.
Productivity of rAAV2/8-GFP
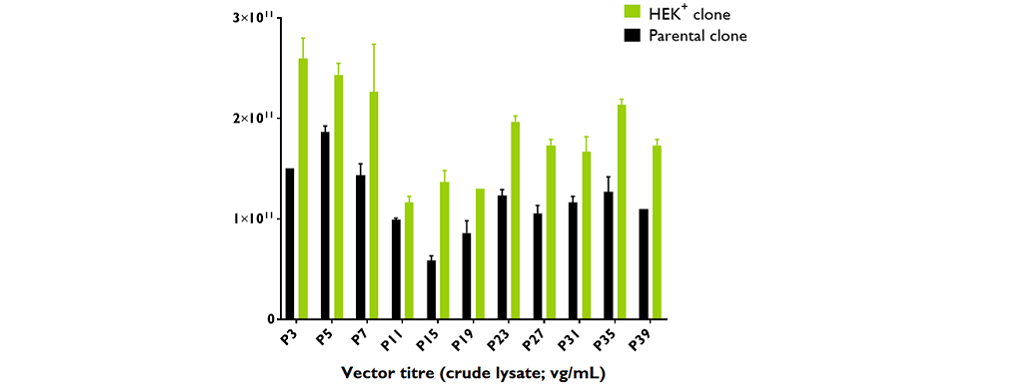
The rAAV2/8-GFP was produced in triplicate at 100mL scale in 250 mL shake flasks between passage 3 and 39. The productivity, based on qPCR titre is significantly higher than the parental clone regardless of the passage number.
Conclusion
- Improved rAAV productivity with HEK+ cells regardless each serotype at both small and large process scale;
- HEK+ cells are stable up to P39 without SV40 T antigen expression and with similar E1A and E1B mRNA expression levels and higher rAAV productivity than observed with the parental clone.
In summary, we have generated a “GMP Master Cell bank” for this new cell clone “HEK+” which can be used to effectively produce rAAVs with a serum-free suspension process without SV40 T antigen expression.


