AAVelocity™: Plug & Play Proven and Flexible, AAV Manufacturing Platform
AAV-based process platform
Adeno-associated virus (AAV) is one of the most common viruses used as a viral vector in cell and gene therapies. This non-integrating vector offers many advantages as a viral vector for cell and gene therapies and AAV-based therapies are set for “greater approval number and approval of treatments for more prevalent diseases”, making it the most represented viral vector within therapies starting in 2025. “By 2028, in fact, sales of AAV-associated cell and gene therapies are projected to be 72% higher than LV-based therapies” (Source: Cell and Gene Therapy: 2023 Market Analysis, CDMO Pricing and Benchmarking, niceInsight).
Having that in mind, we have developed AAVelocity™: a unique and flexible AAV manufacturing platform for high-yield, time-saving, and scalable AAVs for your cell and gene therapies.
Using a HEK-293 cell line and a proprietary optimized transfection reagent, our AAV platform, AAVelocity, is compatible with suspension systems, using shake flasks and single-use bioreactors, and is streamlined to reduce time-to-market for your cell and gene therapy products.
An agile platform
Platforms can be agile, indeed, AAVelocity was designed by our experts to be a Plug-and-Play, still flexible platform for your AAV manufacturing needs. The goal behind that is to have a proven yet agile platform with steps that can be added or removed for time and cost-effectiveness at its climax.
Are you curious to know which steps are flexible? Contact us to learn more about the agility of AAVelocity!
A fully integrated AAV platform
Manufacturing your AAV with AAVelocity, you will have access to a fully integrated platform. Because everything is optimized and set up with efficient procedures, only 12 months are needed from the plasmid availability to the release of the first clinical cGMP batch of your project. Our Experts have tested and improved AAVelocity to produce premium viral vectors for your cell and gene therapies.

A scalable and highly productive AAV platform
AAVelocity has been developed to be scalable and highly productive.

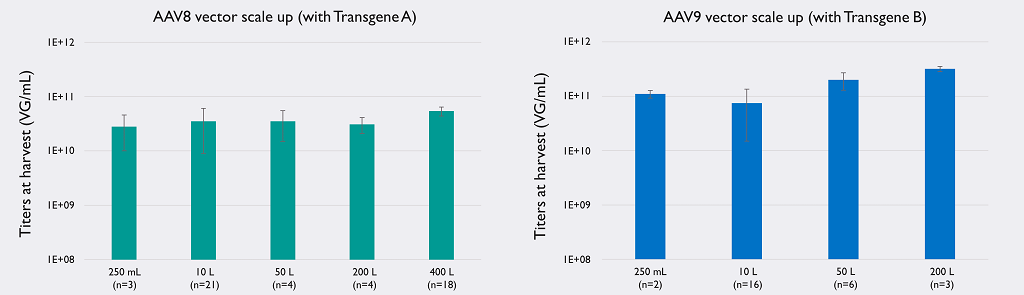
Indeed, AAVelocity is equipped with bioreactors up to 1,000 L and delivers yields at harvest up to 2×1011 VG/mL (vector dependent) with a percentage of full particles higher than 80% and a total yield between 30-50%, making it one of the most powerful AAV platforms on the market.
On top of that, our experts have gained experience with several serotypes and capsid variants.
An experienced, robust, and safe AAV platform
With more than 55 batches of current Good Manufacturing Practices (cGMP) AAV already produced, our highly qualified experts have the knowledge and experience needed to manufacture AAVs in a safe and cost-effective manner.
With a high success rate of >95%, the AAVelocity platform is robust and designed to produce high-quality grade AAVs.
The patient being at the heart of our manufacturing development, we take safety very seriously and have a proven Contamination Control Strategy (CCS).
About the cell line
Because innovation and constant improvement are one of our main concerns, we have developed “HEK+” cells, SV40 large T antigen encoding sequence deleted cell lines for our AAV platform. Coupled with a serum-free suspension process, the HEK+ cells offer stability and improved yields from our platform. Indeed, the HEK+ cell line improves the AAV productivity, regardless of the serotype at both small and large process scales and HEK+ cells are stable up to P39 with consistent performance, and without SV40 T antigen expression and with stable E1A and E1B mRNA expression levels.
Expertise
The experts behind AAVelocity make the platform effective, we have been producing AAV for over 30 years. Numerous successful AAV batches have previously been delivered by our outstanding of highly qualified experts.
AAVelocity is optimized and automated, to make the following promises:
- Decreased number of staff interventions
- Reduced costs
- Increased efficiency
Analytics
Because AAV manufacturing and analytics are inextricably linked, AAVelocity is equipped with proven and optimized analytical methods. Recognized by the leading regulatory authorities, 95% of our analytical tests are performed in-house and a fully qualified external network is well-established for specialized testing. Click here to find out more about our analytical methods.
Frequently Asked Questions (FAQs)
The manufacturing of AAVs involves several steps:
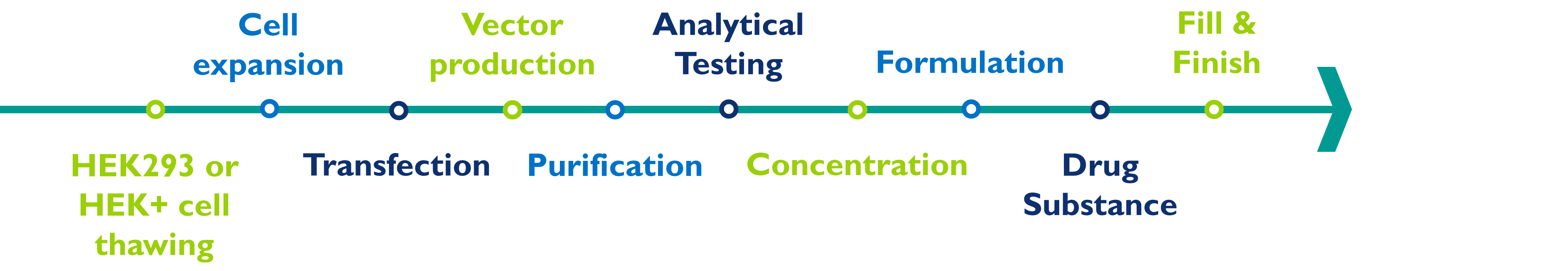
HEK293 or HEK+ cell thawing and expansion: To thaw and then expand the number of cells in the culture, this step can be done with an adherent or a suspension bioreactor.
Vector production: The AAVs are transfected into cells, which allows the production of large quantities of AAVs particles. This is usually done in multi-layer cell culture flasks or suspension bioreactors using chemical transfection reagents.
Virus purification: The AAVs particles are then purified from the cell culture media using techniques such as filtration, chromatography, and tangential flow filtration.
Analytical testing: The purified AAV is then tested to ensure that it is pure and free of contaminants. This can be done using techniques such as biochemical assays, molecular biology tests, protein assays, and potency assays.
Virus concentration: The purified AAV is then concentrated to the desired concentration using techniques such as ultra- or tangential flow filtration.
Formulation: The concentrated AAV is then formulated with excipients.
Drug substance: The final formulated bulk drug substance can be frozen and stored for later drug product manufacturing or can be onward processed to the drug product manufacturing step.
Fill and finish: As a last step, the formulated concentrated AAV is packaged into appropriate containers for storage and use. It is important to follow cGMP when producing AAVs to ensure that the final product is safe and effective for use in humans.
Yes, AAVelocity was developed using single-use equipment, except for certain chromatography resins which are packed into reusable columns. Working with single-use equipment allows our experts to work in a closed environment, reducing the risk of cross-contamination, and providing consistent batch-to-batch results. On top of that, it allows our scientists to work on a fast change-over time between batches, improving the cost-efficiency of our AAV platform.
Yes, to avoid variability and contaminants in cell culture systems, the AAVelocity suspension platform uses serum-free media, and no animal product is used within the platform. Using defined components media allows us to manufacture AAVs with consistency and contamination control.
When a cell and gene therapy developer wants to access the AAVelocity platform, we will start with a standard feasibility study to optimise productivity, followed by the manufacturing of a technical batch and clinical batch, with associated analytics.
Yes, a platform for lentivirus manufacturing, LentiSure™ is also available. Learn more about the lentivirus platform here.
Yes, because we know that science is constantly evolving, we are always working on innovating and improving our platforms. Learn more about our innovation programs by clicking here.


