AAV and lentiviral vector production specialized CDMO
Yposkesis’ Services
Yposkesi is a viral vector manufacturing Contract Development Manufacturing Organization (CDMO) providing professional support through all stages of cell and gene therapy development. Because we know that time is of the essence in the clinical development and commercialization of new therapies, we’ve developed a full-service viral vector approach for your cell and gene therapies.
Leveraging our 30 years of experience, we can help reach your goals from analytical and process development through to commercial production.
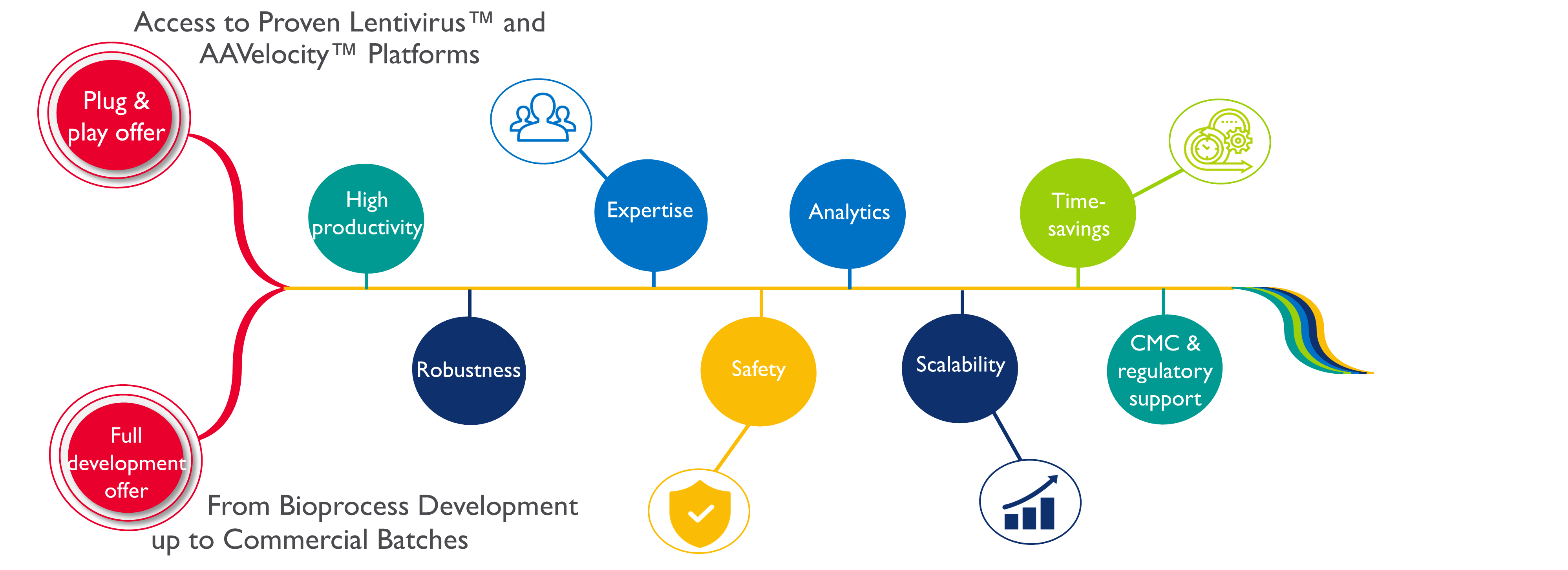
- With specific manufacturing platforms: Developed over the years, our Adeno-Associated Virus (AAV) and Lentiviral vectors, AAVelocity™ and LentiSure™, platforms benefit from high-producer cell lines and a proprietary optimized transfection reagent. Click here to learn more about our platforms;
- With multiple Analytical and Process Development Laboratories: We offer flexibility to develop the best process and analytical methods depending on your needs. Your Cell and Gene therapy product being unique, the analytics and manufacturing process for viral vector will be customized to your needs;
- With a total of six independent cGMP manufacturing suites available to develop your Advanced Therapy Medicinal Products (ATMPs):
- Suspension systems: cGMP total capacity of 7,000 L with bioreactors from 50 L up to 1,000 L;
- Adherent systems: cGMP capacity of 24 x CF10;
- With two cGMP aseptic and semi-automated fill and finish suites:
- Total capacity of 12,000 vials per year;
- Using a variety of cGMP compliant vials filling formats, including Crystal® Closed Vial Technology (Cyclo-Olefin Co-polymer) and Glass vials;
- We are currently working on the implementation of a fully automated Drug Product fill & finish line.
In order to optimize efficient multi-product manufacturing, our processes use single-use equipment.
A bespoke service
As each project is unique, we will develop with you the best processes for your cell and gene therapy manufacturing and will accompany you during the development. Whether you have a full development or plug-and-play project, our experts and viral vector manufacturing platforms are ready to partner with you.
Project management
From the beginning of your project, a dedicated Chemistry, Manufacturing, and Control (CMC) project manager will be assigned to you, becoming your key point of contact at Yposkesi. Ensuring you are kept up to date with every step of project progress, and you get real time updates from Yposkesi’s dedicated team of experts. It is their responsibility to assure a project is on track for timelines, quality requirements, and costs.
The project manager will:
- Organize a kick-off meeting: to review your project content (work packages definition with technical content, pre-requisites, expected outcome, deliverables, milestones, etc), timeline, presentation from the dedicated Yposkesi teams, and if necessary, present a risk assessment plan.
- Organize regular team meetings: frequency is adapted depending on your project phase and needs. During each project team meeting, driven by the project manager, a project update is performed with a review of each work package for ongoing and next steps including technical content, timelines, and quality.
As the right partner to bring your cell and gene therapies to life, we took the accelerated path to commercial production. We are currently building on our existing campus, a new state-of-the-art facility that will increase our current manufacturing footprint from 50,000 sq ft. (approx. 5,000m2) to 100,000 sq ft. (approx. 10,000m2), to further ensure that you meet your timelines and budget.
Discover our services

Bioprocess development (USP & DSP)
We use innovation to reach new heights in production efficiency.
Discover
cGMP manufacturing of clinical and commercial viral vector batches
Our extensive AAV and lentiviral vector manufacturing capacity helps ensure on-time deliver.
Learn more
Analytical development, assays qualification & validation
Our experts aim to satisfy all your analytical needs with high quality.
Discover
Fill & Finish
We offer a variety of cGMP compliant vials filling format for Fill & Finish
Learn moreFrequently Asked Questions (FAQs)
A Contract Manufacturing Organization (CMO) is a type of CDMO specializing in manufacturing. A CMO does not offer development services; instead, they focus on large-scale commercial manufacturing of pharmaceutical and biopharmaceutical products.
As a full-service CDMO, Yposkesi is offering the development as well as the manufacturing of AAV and lentiviral vectors for your ATMPs.
There are many benefits of working with a CDMO, including:
- Access to experts: CDMOs employ experienced professionals who can provide expert guidance and support throughout the product development process;
- Flexibility: CDMOs can be engaged on a project-by-project basis, which gives pharmaceutical companies the flexibility to scale up or down as needed;
- Cost-effectiveness: CDMOs can often provide services at a lower cost than in-house development and manufacturing;
- Improved time to market: CDMOs can help reduce the time it takes to bring a product to market.
When choosing a CDMO partner, it is vital to consider the following factors:
- Service offering: Does the CDMO offer the services you need?
- Expertise: Does the CDMO have expertise in the relevant therapy area?
- Capacity: Does the CDMO have the capacity to meet your needs?
- Location: Is the CDMO located in a convenient location?
- Cost: Is the CDMO competitively priced?
- Reputation: Does the CDMO have a good reputation?
If you are looking for a full-service CDMO with cell & gene therapy expertise, look no further! With more than 30 years of experience and services from bioprocess development up to commercial manufacturing, Yposkesi can help you bring your product to market quickly and efficiently.
Yposkesi offers a comprehensive range of services to support your clinical trials and commercial production, including:
- Bioprocess Development: We are fully equipped to conduct small-scale to large-scale manufacturing of your product using our proprietary platforms and technology, increasing production efficiency and reducing costs;
-
cGMP Manufacturing of Clinical and Commercial Viral Vector Batches: We have four independent cGMP manufacturing suites that can execute adherent or suspension production processes. These suites are designed to efficiently produce large-scale clinical and commercial batches of viral vectors. This ensures that the production is on time, on budget, and to the highest quality standards;
- Analytical Development, Assays Qualification & Validation: We aim to provide products and services of the highest quality. Our analytical development processes are designed to ensure that the products we manufacture meet the highest quality standards;
- Fill & Finish: We offer aseptic vial filling services for your ATMP products. Our selection of cGMP-compliant vials is tested and qualified to ensure product safety, efficacy, and integrity;
- Regulatory Support: We provide comprehensive regulatory support services throughout the clinical development process. We will work with you to develop a clinical trial design that is efficient and compliant with all relevant regulations.
Contact us to learn more!



